1. General introduction
The scope of disinfection in China is the treatments that kill or eliminate pathogenic microorganisms on the transmitting vector and making it harmless. Disinfectant products can be classified according to application for drinking water-related area, veterinary area, and others (e.g. fruits and vegetables, tableware and food contact materials, human skin/mucosa, environment and object surface, air, medical articles, chemical indicators). Currently, the regulatory administration on disinfectant products includes the National Health and Family Planning Committee (NHFPC) for food and non-food related products and Ministry of Agriculture (MOA) for veterinary disinfectant. The management of water-related and others are different. The disinfectant products that covered in this article focus on the others area. The Chinese government started to regulate disinfectant products since 1987 with hygiene licenses. In the year of 2000, the authority requires all disinfectant products much have final review by former Ministry of Health (MOH). But after that, the Ministry of Health excluded registration requirements on unnecessary product types step by step to make the whole management more scientific. Not until 2011, the MOH issued administrative approval measures for disinfection products and drinking water-related products, and in 2014 the NHFPC issued Regulations on Administrative License of New Disinfection and Aquatic Products, the comprehensive regulatory system is finally established as we know today. Through the regulation reform, disinfectant management has transferred from a strict and all-encompassing pre-market approval to an increased focus on post-market supervision.
Ministry/Administration Involved in Disinfection Regulation
Ministry/Administration | Regulatory Duties |
National Health and Family Planning Commission (MOH) | - draw up related regulations, policies, plans, standards and technical specifications; - evaluate new disinfection product registration and issue registration approval. |
Provincial Health Administrative Departments | -evaluate and approve existing product notification application; -being responsible for land investigation and product sampling. |
Chinese Disinfection Legislative Framework
Regulations | Issuing Body | Scope |
Measures on Administration of Disinfection Products | National Health and Family Planning Commission | -the essential measures to regulate disinfections production and sales; -contains sanitary standards for sterilization; -production and management requirements of disinfection product; -regulation for sterilizing institutions and penalty. |
Criteria for Determining of Disinfectants and Disinfecting Devices Produced by the Use of New materials, New Technology and New Principle of Sterilization | National Health and Family Planning Commission | -to sift and determine the regulatory compliance requirement for disinfectant products |
Regulations on Hygiene and Safety Assessment of Disinfectant Products | National Health and Family Planning Commission | -requires that responsible units conduct the hygiene and safety assessment for disinfectants, disinfecting devices and anti-bacteria agents, and other existing disinfection products; -guarantee the hygiene and quality of the products. |
Regulations on Administrative License of New Disinfection and Aquatic Products | National Health and Family Planning Commission | -clearly stipulate the requirements of content, time limit, application acceptance criteria, examination and determination; -details the application procedures, documents and other materials required. |
Compliance responsibility
Generally, currently, there are two compliance requirements refer to disinfection products, one is registration of new disinfection products and the other is hygiene and safety assessment of non-new disinfectant products and filing proper records with the competent health administrative agency. According to the regulation issued by NHFPC, new disinfectant products are mainly referred to products that apply new materials, new processes and technologies, and new sterilization principles, which are also called the “three new types of disinfectants”.
Responsible party
First of all, any disinfectant product compliance should be conduct by a responsible party within China mainland. Especially for those foreign exporters, they should have a Chinese responsible party for their product recording or registration. It can be its distributor or branch office.
Non-new/Existing disinfectant products
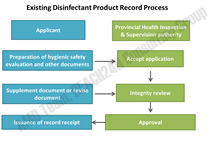
Decades ago, all disinfectant products were required to absorb approval license, while, after a series of regulation reform, the safety assessment accountability of existing disinfectant products has moved from the government to manufacturers. Products that fall in the scope of existing disinfectant product are required to do self-assessment by companies themselves. Based on the different risk levels of the products, the compliance requirement differs. Existing disinfectant products are classified into 3 categories based on the product application area and risk level:
Classification | Category 1 (relatively high risk) | Category 2 (medium risk) | Category 3 (relatively low risk) | |
Products |
|
|
| |
Compliance requirement | Hygienic safety assessment and recoding in provincial level | Meeting label requirement | ||
Validity period | 4 years | No expiration data | No expiration data | |
Note: When the same disinfectant product involves different categories, it shall be regulated as the category of higher risk.
For a disinfectant product in either Category 1 or Category 2, the responsible party must conduct a hygienic safety evaluation before marketing for the first time. This evaluation is to be conducted either by the responsible party or by entrusting a third party. A disinfectant product can only be marketed after being qualified by the hygienic safety evaluation.
Fig.1 -Existing Disinfectant Product Record Process in China
Hygienic safety evaluation should include:
product labels (nameplates) ;
user directions;
test reports (including the result);
enterprise standards or quality standards;
hygienic licenses for domestic products;
production and sales permit in the producing country (region) for imported products;
product formula evaluation for disinfectants, biological indicators, chemical indicators, packaging for sterilization articles with sterilization mark and antimicrobial (bacteriostatic) agents;
main product components and structure drawings evaluation for disinfecting devices.
Tests shall be conducted in qualified disinfectant product testing institutions.
New disinfectant products
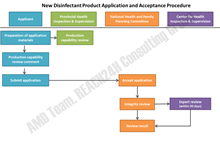
Before a new disinfectant product enters Chinese market, it should submit a new disinfectant product application and get approval from the NHFPC. Whether a product is in the scope of “three new types of disinfectant products” is sometimes difficult to determine, especially for new developed products. Normally, new disinfectant products should have equivalent or better performance comparing to existing products in terms of effective ness, safety and environmental adaptability. In general, the registry process is very clear. To registry “three new types of disinfectant products”, companies should prepare and submit application materials, and then the experts of NHFPC shall conduct a risk assessment review, and the outcome will be publicized.
Fig.1 -New Disinfectant Product Application and Acceptance Procedure
Application materials include:
hygiene administrative approval application form for new disinfectant;
outcome of assessment on production capacity by a provincial-level health supervision agency;
research and production report;
description of features of the new product;
formula or structure chart;
introduction and sketch of production processes;
corporate standard or product quality standard;
product description, label or plate;
certificate for production and sales of producing state or region (for imported new disinfectants);
letter of authorization for a liability entity in China (for imported new disinfectants);
letter of entrustment for application (if entrustment is needed);
other possibly supporting document.
It should be noted that the expert review committee need applicants to answer questions to verify the result or method of testing in the application. And for those foreign companies that apply the new disinfectant products for first time in China, the authority demands to collect sample by personnel sent by local health administrative agency.