Born in the wake of elevated concern about environmental pollution, the United States Environmental Protection Agency (EPA) was established on December 2, 1970 to consolidate in one agency a variety of federal research, monitoring, standard-setting and enforcement activities to ensure environmental protection. Since its inception, EPA has been working for a cleaner, healthier environment for the American people.
Pesticides must be registered with EPA unless they meet the criteria for a minimum risk pesticide. EPA evaluates pesticides to ensure that when they are used according to label directions they will not harm people, non-target species or the environment. Companies are required to submit to EPA for review information about the health effects of pesticides. EPA also funds pesticide research engaging the nation's best scientists and engineers to improve knowledge about how they are exposed to pesticides and their health effects. Once registered, pesticides are periodically reviewed for safety. If new concerns arise, EPA can change the conditions for using them or cancel their registrations.
It is illegal to use a pesticide product inconsistent with its label directions.
What is a pesticide?
A pesticide is any substance or mixture of substances intended for
Preventing, destroying, repelling or mitigating any pest.
Use as a plant regulator, defoliant, or desiccant.
Use as a nitrogen stabilizer
Federal Pesticide Laws
Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA)
- Requires all pesticides sold or distributed in the United States (including imported pesticides) to be registered by EPA.
- Registration is based on evaluation of scientific data and assessment of risks and benefits of a product's use.
- Label directions control how products are used.
- Authorizes limited use of unregistered pesticides or pesticides registered for other uses to address emergencies and special local needs.
- suspends or cancels a product's registration.
- Training is required for workers in pesticide-treated areas and certification and training for applicators of restricted use pesticides.
Federal Food, Drug and Cosmetic Act (FFDCA)
Requires EPA to set pesticide tolerances for all pesticides used in or on food or in a manner that will result in a residue in or on food or animal feed. A tolerance is the maximum permissible level for pesticide residues allowed in or on human food and animal feed.
Includes strong provisions for protecting infants and children, as well as other sensitive subpopulations.
Provides for exemption from the requirement for a tolerance.
Under the Food Quality Protection Act (FQPA), of 1996, which amended both FIFRA and FFDCA, EPA must find that a pesticide poses a "reasonable certainty of no harm" before it can be registered for use on food or feed. EPA must review each pesticide registration at least once every 15 years.
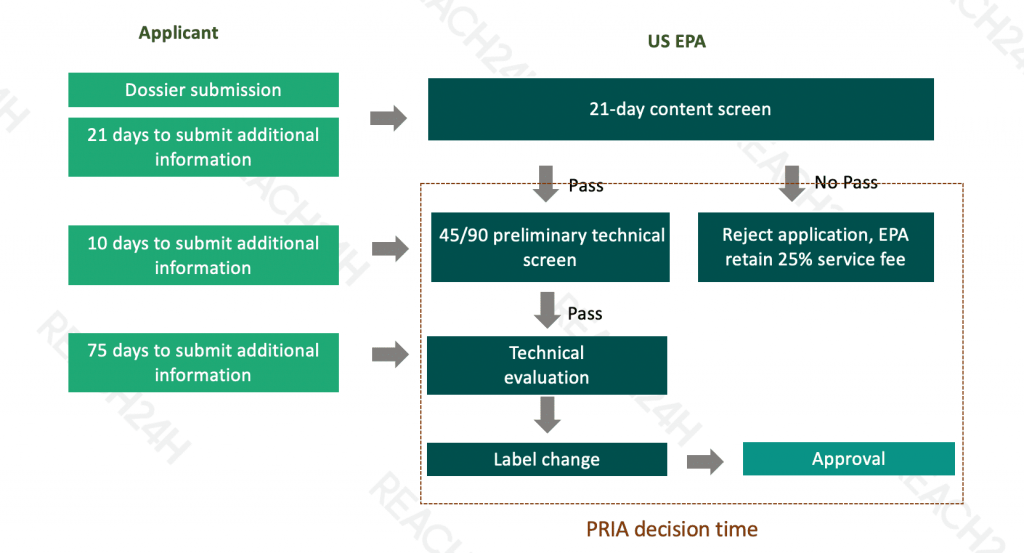
The Pesticide Registration Improvement Act of 2003 (PRIA) also amended FIFRA and FFDCA. PRIA fees have been reauthorized three times, most recently by the Pesticide Registration Improvement Extension Act of 2018 (PRIA 4). This reauthorization expires September 30, 2023. Under PRIA:
Companies must pay service fees according to the category of the registration action.
EPA must meet decision review time periods, which result in a more predictable evaluation process for companies.
Shorter decision review periods are provided for reduced-risk registration applications.
The fees and decision review periods may change from one fiscal year to the next, and fees are periodically increased as prescribed by statute. Applicants should refer to the fee determination decision tree or fee table for assistance in finding the appropriate PRIA category and fee for their submission.
The Endangered Species Act (ESA) requires federal agencies to ensure that any action they authorize, fund, or carry out, will not likely jeopardize the continued existence of any listed species, or destroy or adversely modify any critical habitat for those species. EPA is responsible for reviewing information and data to determine whether a pesticide product may be registered for a particular use. As part of that determination, EPA assess whether listed endangered or threatened species or their designated critical habitat may be affected by use of the product. All pesticide products that EPA determines “may affect” a listed species or its designated critical habitat may be subject to EPA's Endangered Species Protection Program.
Types of Pesticides
Conventional pesticides
Antimicrobial pesticides
Biopesticides
Inert ingredients
Types of Registrations under FIFRA
Federal pesticide law (the Federal Insecticide, Fungicide, and Rodenticide Act) provides for several types of registrations:
Registration: Under Section 3 of FIFRA, EPA can register pesticides for use throughout the United States. We register some pesticides for more limited use in certain states. In addition, states, tribes and territories can place further restrictions on pesticides used or sold within their own jurisdictions.
Experimental Use Permits (EUPs): Under Section 5 of FIFRA, EPA can allow manufacturers to field test pesticides under development. Manufacturers of conventional pesticides must obtain experimental use permits before testing new pesticides or new uses of pesticides if they conduct experimental field tests on 10 acres or more of land or one acre or more of water. Biopesticides also require EUPs when used in experimental settings.
Emergency Exemptions: Under Section 18 of FIFRA, EPA can allow state and federal agencies to permit the unregistered use of a pesticide in a specific geographic area for a limited time if emergency pest conditions exist. Usually, this arises when agricultural growers and others encounter a pest problem on a site for which there is either no registered pesticide available, or for which there is a registered pesticide that would be effective but is not yet approved for use on that particular site. Also, exemptions can be approved for public health and quarantine reasons.
State-Specific Registrations: Under Section 24(c) of FIFRA, states can register a new pesticide product for any use, or a federally registered product for an additional use, as long as there is both a demonstrated "special local need," and a tolerance, exemption from a tolerance, or other clearance under FFDCA. We can disapprove a state's special local need registration.
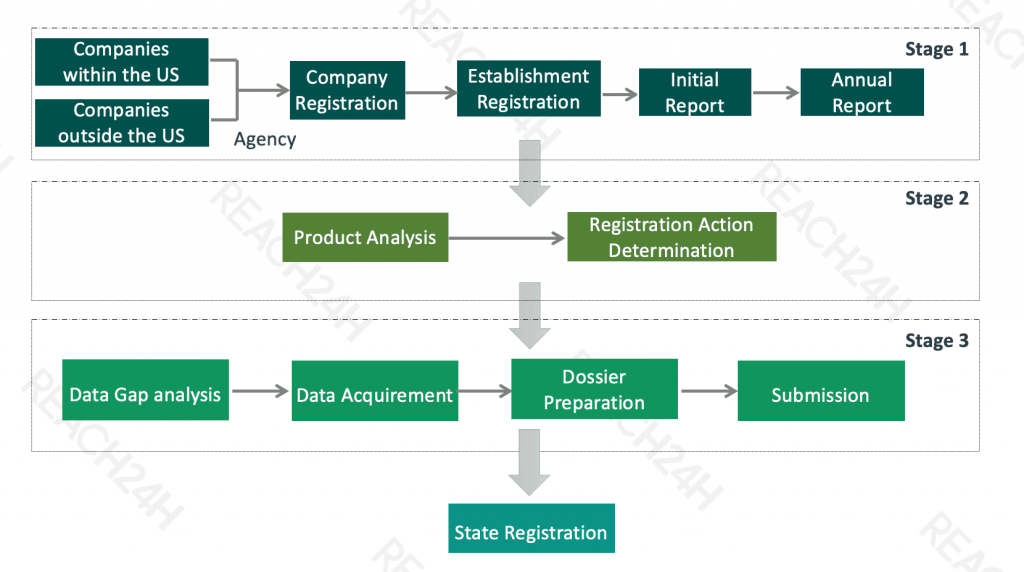
Who Should Register?
Pesticide registration is not only an obligation of pesticide manufacturers, traders, distributors, but pesticide users can also apply for EPA pesticide registration if necessary.
Besides, foreign companies must designate a U.S. agent to receive correspondence and represent them in matters concerning their application.
Compliance Obligations
Obtain a company and establishment number
Submit an initial report and annual report for pesticide-producing establishment
Federal product registration
State product registration
Annual maintenance fee of registration certificate
Registration Exemptions
Treated articles
Pheromones and pheromone traps
Preservatives for biological specimens
Foods
Natural cedar
Minimum risk pesticides
Federal Pesticide Registration
Registration Process
Registration Action Code Determination
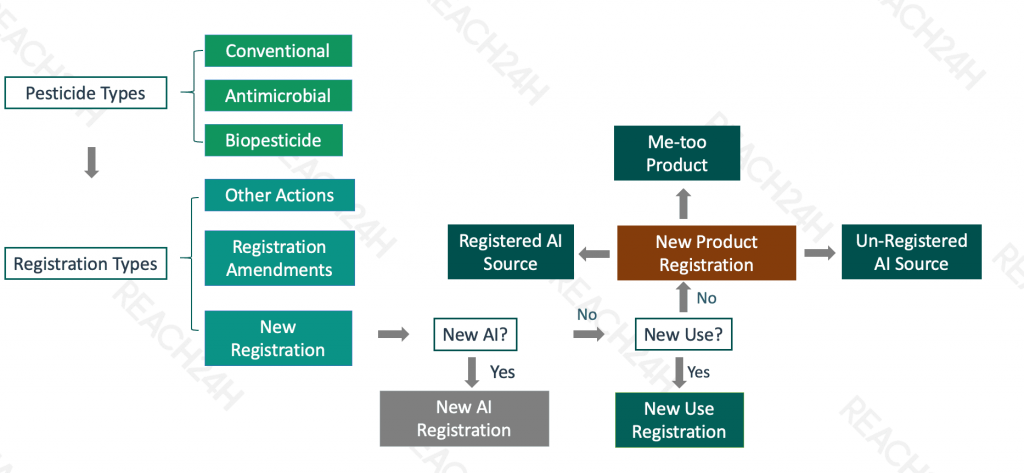
Me-too Registration
The simplest type of US pesticide registration is me-too product registration, or identical/similar product registration, which is determined on the following basis:
| Content | Identical Product | Similar Product |
| Active Ingredients | Identical | Identical |
| AI content | Identical | Substantially Similar |
| Form | Identical | Identical |
| Inert Ingredients | Identical | Substantially Similar |
| Use | Identical | Identical or less |
| Risk | Identical | Substantially Similar |
| Label | Administrative changes | Substantially Similar |
Me-too product registration is a short and cost-effective type of registration compared to other registration types, which can cost hundreds of thousands of dollars and take up to two years to review. In addition, new product registration, other than new use, or new active ingredient, is also a cost-effective type of registration.
Review Procedures
Registration Service Fee Waivers
An applicant that qualifies as a small business is eligible for a partial waiver of 50% or, in some cases, 75% of the registration service fee. The fee waivers apply to both domestic and foreign registrants. A small business means a corporation, partnership, or unincorporated business that fulfill the requirements below:
| If your number of employees is | If your average annual revenue from pesticides is not greater than | You are eligible for a waiver of |
| ≤500 | $60 million | 50% |
| ≤500 | $10 million | 75% |
Re-registration/Registration Review
EPA reviews each registered pesticide at least every 15 years to ensure that each pesticide can carry out its intended function(s) without creating unreasonable adverse effects to human health and the environment. While each pesticide review is unique, all pesticides go through the same basic registration review process.
If a new assessment of the pesticide and additional data are needed to conduct the review, EPA issues a Data Call In (DCI).
Data Protection
To protect the interests of registrants, EPA protects data used for pesticide registration in ways that depend on the type of data.
Exclusive Use Data
Data submitted after September 30, 1978 to the Agency in support of the initial registration of a new active ingredient, a new combination of active ingredients, or in support of an application to amend the original registration to add a new use.
These data have “exclusive-use” protection for a period of 10 years after the date of the initial registration. The Agency cannot consider these data in support of an application for registration or amendment to a registration unless written authorization from the original data submitter is obtained authorizing the Agency to use these data to support the application.
Compensable Data
Compensable data are data submitted to support an application (including reevaluation and DCI data) and no longer retain exclusive-use protection.
These data have a 15-year compensatory period beginning on the date the data were originally submitted to EPA. As long as the applicant/registrant relying on these data has certified to the Agency that it has offered to pay compensation to the original data submitter(s), the Agency may, without the permission of the original data submitter, consider these data in the registration review.
Other Data
Besides these two categories. All other data including public literature articles, government-generated studies, and data for which all periods of exclusive use and compensation have expired, may be cited by an applicant without permission or an offer of compensation.